NH3 has one atom of nitrogen hence the lack of subscript after the N in NH3 and three atoms of hydrogen hence the 3 after the H in NH3. How many atoms does 2 ci2 have.
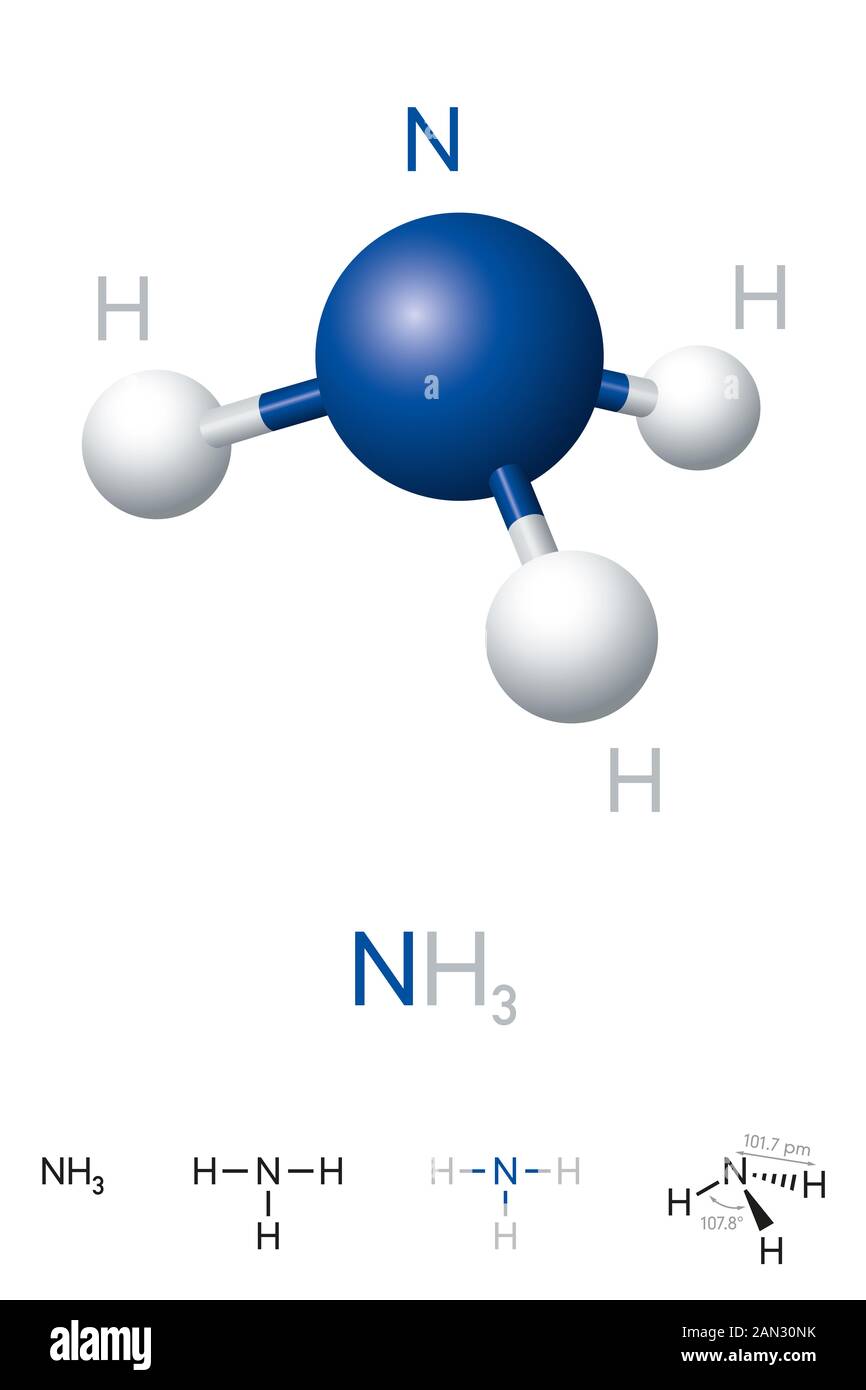
Ammonia Nh3 Molecule Model And Chemical Formula Chemical Compound Of Nitrogen And Hydrogen A Colorless Gas Ball And Stick Model Stock Photo Alamy
There are total 4 atoms in ammonia.

. Ammonia NH3 is a chemical compound composed of one nitrogen atom and three hydrogen atoms. Compossed of three hydrogen atoms and one nitrogen atom. 8 clever moves when you have 1000 in the bank.
The Lewis dot structure for ammonia NH3. Experts are tested by Chegg as specialists in their subject area. In 1 mole of ammonia 1 atom of nitrogen is there and 3 atoms of hydrogen is there.
Has a triangular pyramidal geometry together with a boiling point of 777C. How many nitrogen atoms and hydrogen atoms are there. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
According to an industry convention the numbers on the label refer to the mass percents of n p2o5 and k2o in that order. In one molecule of ammonium nitrate there are 9 atoms 2N 4H 3O so only 29 of the 30093741 x 1020 will be nitrogen atoms 30093741 x 1020 x 29 6687 x 1019 atoms of Nitrogen in 00400g of ammonium nitrate. 1 mole is 60221023 atoms.
One atom of nitrogen and 3 atoms of hydrogen. The small number after the element symbol is called the sub. 1 Get Another question on Chemistry.
If we multiply these numbers by 2 we can also say you need 2 moles of nitrogen and 6 moles of hydrogen to make 4 moles of ammonia. 79 mol of nh3. In one molecule of NH3 there is 1 atom of N and 3 atoms of H for a total of 4 atoms.
The number of atoms in NH3or atomicity of NH34 AS we can clearly see that ammonia contains 2 elements namely hydrogen and sodium and also 3 after hydrogen depicts that there are 3atoms of hydrogen in amm. This molecule is formed by the combination of hydrogen and nitrogen atoms. This is a statement i saw from a website.
So 3 atoms of nitrogen is there and 9 atoms of hydrogen is there. You need 1 mole of nitrogen and 3 moles of hydrogen to make 2 moles of ammonia. Currently we have 2 atoms of nitrogen and 2 atoms of hydrogen on the reactant side and 1 atom of nitrogen and 3 atoms of hydrogen on the product side.
Who are the experts. How many nitrogen atoms are in 400 g of NH3. If we look at 1 NH3 1 mol NH3 or 1 molecule NH3 we can see that 1 molecule NH3 has 1 atom of N and 3 atoms of H.
How many atoms is 3nh3. How many atoms in nitrogen atoms in 2. How many atoms are in a element.
There are 9 hydrogen atoms in the given chemical formula. How many lone pairs does nh3 have Ammonia is a colorless compound used in make fertilizers. Besides how many moles of nh3 are.
See the answer See the answer See the answer done loading. An atom is an element. N mM n is the amount of substance in moles mol.
Thus in two moles the hydrogen atoms is 6. Because the reaction occurs at STP 1 mole of NH3 gas occupies 224 L. NH3 is the chemical formulae of ammonia.
Ammonia NH3 is a chemical compound composed of one nitrogen atom and three hydrogen atoms. This problem has been solved. How many nitrogen atoms are in 400 g of NH3.
The balanced equation indicates that 2 moles of NH3 react to produce 1 mole of NH42SO4. The two words are synonymous so if youre looking for the number of atoms in. To find the total number of atoms in NH3 Ammonia well add up the number of each type of atom.
Ammonia NH3 is a chemical compound composed of one nitrogen atom and three hydrogen atoms. Ammonium nitrate has the chemistry formula NH4NO3 which includes two nitrogen N atoms 4 hydrogen H atoms and also three oxygen O atoms. How do you convert grams to moles calculator.
Nitrogen n phosphorus p and potassium k are the main nutrients in plant fertilizers. How many liters are in 2 moles of NH3. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen the same as occurs in H2O.
Answer is 16810²⁴ N-atoms. And youd have 1 mole of nitrogen left over And thats the answer. M is the mass of the substance in grams g.
How many NH 3 molecules are in 050 mol of NH 3. 1681024 atoms of nitrogen are present in 279 mol of NH3. And 279 mol NH3 have 279 mol of N atoms.
We review their content and use your feedback to keep. Youd get 4 moles of ammonia. How many atoms are present in NH3.
NH3 is Ammonia having one nitrogen atom and three hydrogen atom. One pair The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. How do you calculate N in moles.
We can balance the hydrogens by placing a coefficient of 2 in front of ammonia and a coefficient of 3. N m M where M is the molar mass of this material. 37 Related question Answers Found How carry out you transform from mole to grams.
It is a secure hydride created of one nitrogen and three hydrogen atoms. Also 1 mole of NH3 has 1 mole of N atoms and 3 moles of H atoms.

Ammonia Nh3 As Both Nitrogen And Hydrogen Are Non Metals This Is Covalent Teaching Chemistry Covalent Bonding Science Chemistry
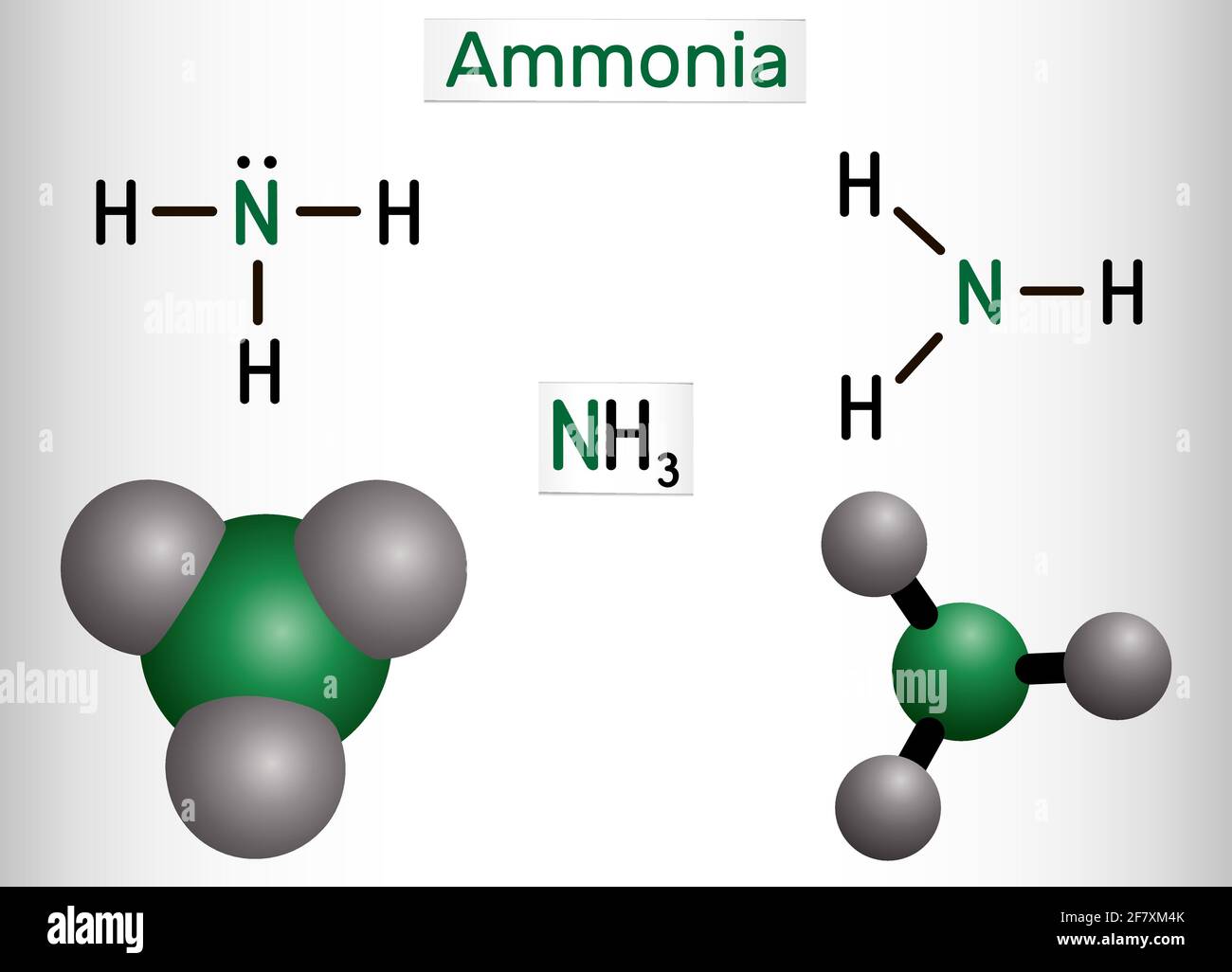
Ammonia Nh3 Molecule It Is Pnictogen Hydride Inorganic Compound Composed Of Single Nitrogen Atom Covalently Bonded To Three Hydrogen Atoms Structu Stock Vector Image Art Alamy

Ammonia Molecule 3d Model 3d Model Molecular Structure Molecules
0 Comments